This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.

Materials and Hydrogen
Outreach at Royce
Hydrogen is an integral part of future sustainable energy solutions for the UK. Materials scientists supported by the Henry Royce Institute are working to develop new systems for renewable and low carbon hydrogen production, storage and distribution so that we are able to decarbonise a range of sectors by the government’s 2050 net-zero target.
Join us at an outreach event where we will be showcasing Materials and Hydrogen and see first-hand how this technology can power a car engine, and find out more about what materials for end-to-end hydrogen means for delivering a sustainable future through the information on this page.
Contact outreach@royce.ac.uk for more information.
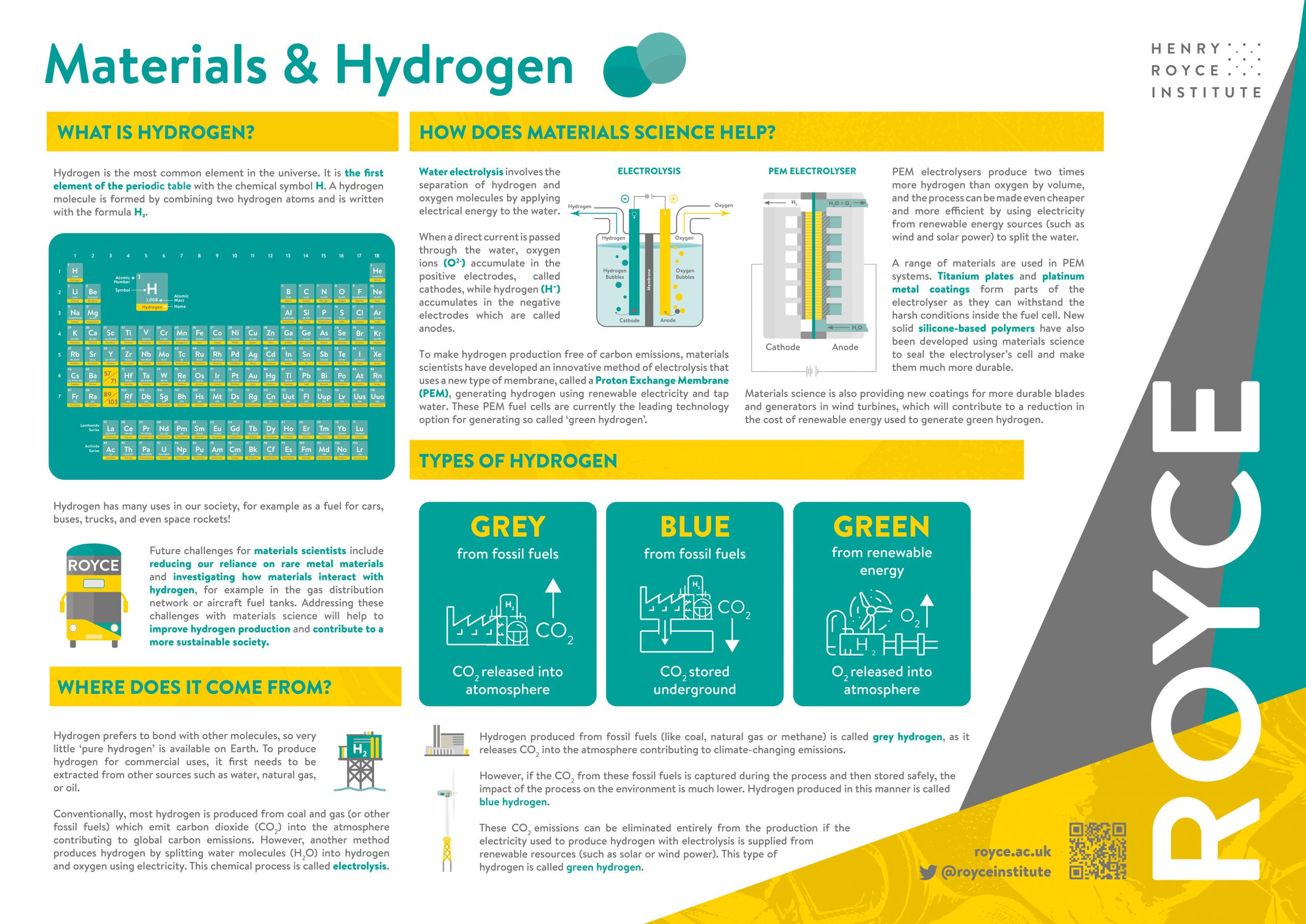
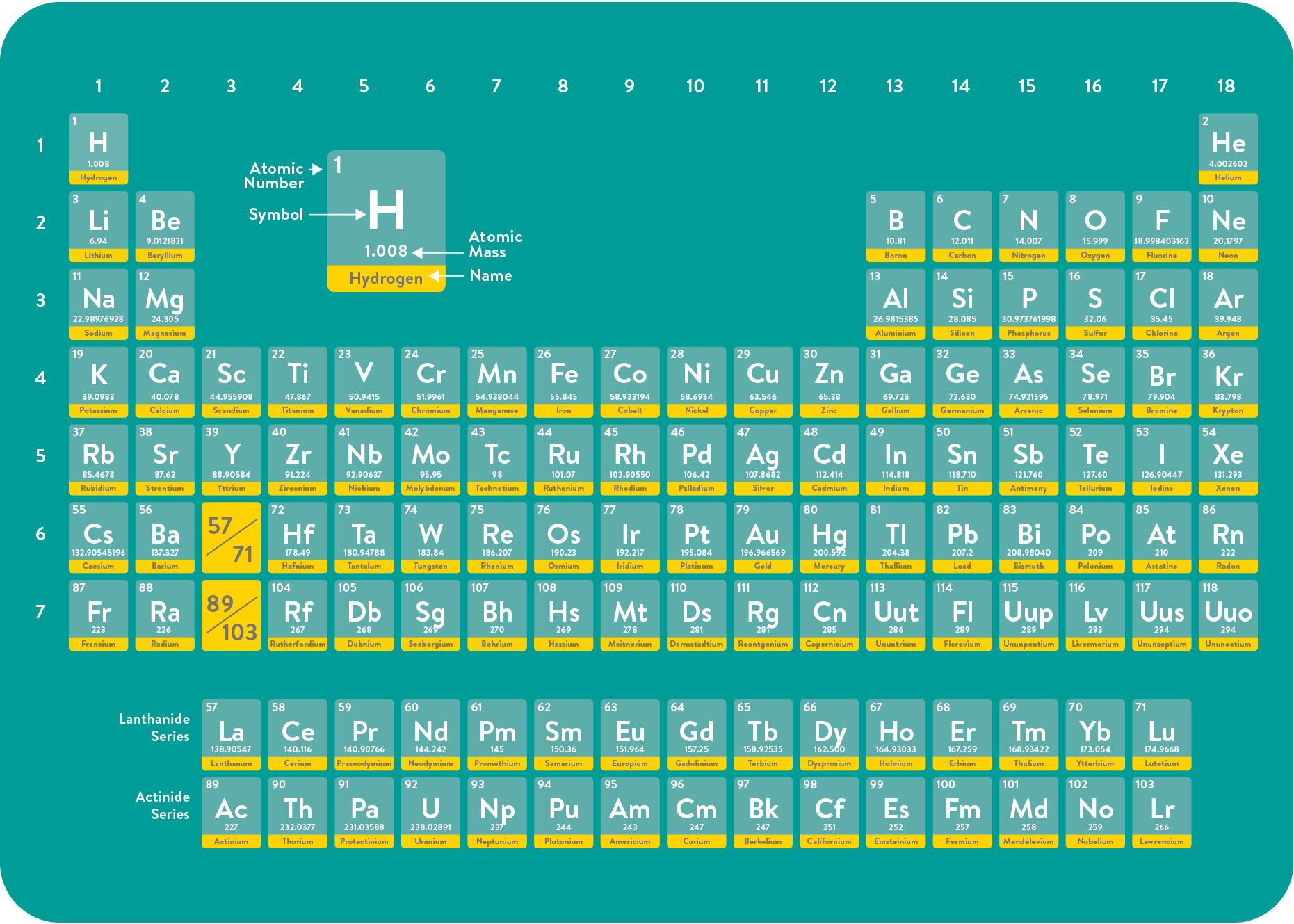
What is Hydrogen?
Hydrogen is the most common element in the universe. It is the first element of the periodic table with the chemical symbol H. A hydrogen molecule is formed by combining two hydrogen atoms and is written with the formula H₂.
Hydrogen has many uses in our society, for example as a fuel for cars, buses, trucks, and even space rockets!
Future challenges for materials scientists include reducing our reliance on rare metal materials and investigating how materials interact with hydrogen, for example in the gas distribution network or aircraft fuel tanks. Addressing these challenges with materials science will help to improve hydrogen production and contribute to a more sustainable society.
Where does it come from?
Hydrogen prefers to bond with other molecules, so very little ‘pure hydrogen’ is available on Earth. To produce hydrogen for commercial uses, it first needs to be extracted from other sources such as water, natural gas, or oil.
Conventionally, most hydrogen is produced from coal and gas (or other fossil fuels) which emit carbon dioxide (CO2) into the atmosphere contributing to global carbon emissions. However, another method produces hydrogen by splitting water molecules (H2O) into hydrogen and oxygen using electricity. This chemical process is called electrolysis.
How does materials science help?
Water electrolysis involves the separation of hydrogen and oxygen molecules by applying electrical energy to the water.
When a direct current is passed through the water, oxygen ions (O2-) accumulate in the positive electrodes, called cathodes, while hydrogen (H+) accumulates in the negative electrodes which are called anodes.
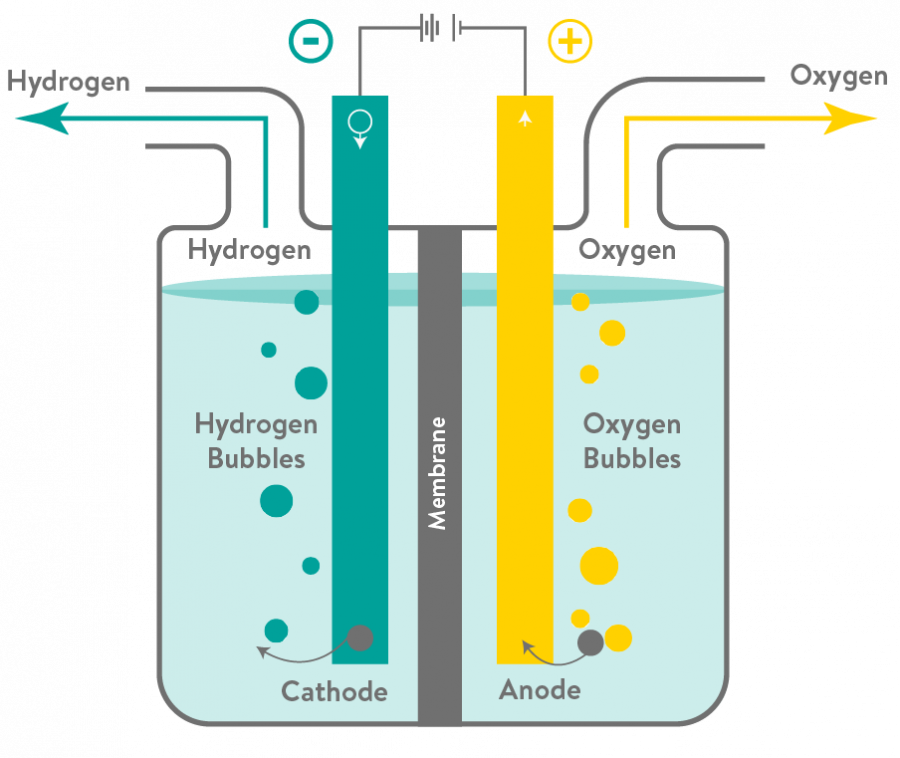
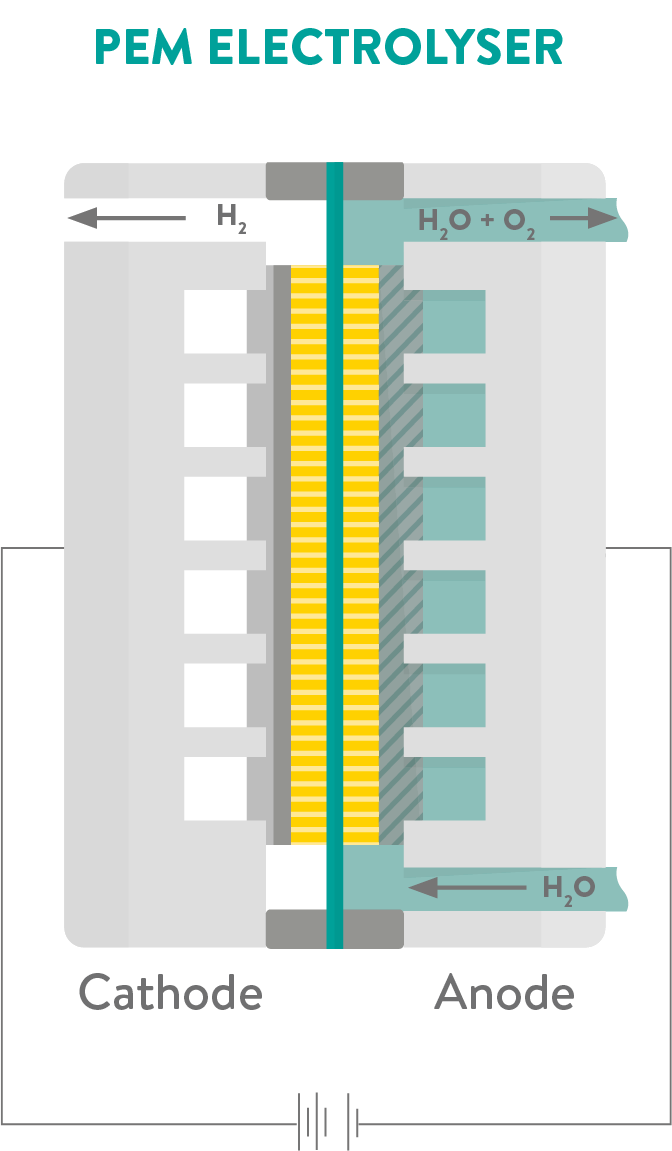
To make hydrogen production free of carbon emissions, scientists developed an innovative method of electrolysis that uses a type of membrane called a Proton Exchange Membrane (PEM), generating hydrogen fuel using renewable electricity and tap water. These PEM fuel cells are currently the leading technology option for generating so called ‘green hydrogen’.
PEM electrolysers produce two times more hydrogen than oxygen by volume, and the process can be made even cheaper and more efficient by using electricity from renewable energy sources (such as wind and solar power) to split the water.
A range of materials are used in PEM systems. Titanium plates and platinum metal coatings form parts of the electrolyser as they can withstand the harsh conditions inside the fuel cell. New solid silicone-based polymers have also been developed using materials science to seal the electrolyser’s cell and make them much more durable.
Materials science is also providing new coatings for more durable blades and generators in wind turbines, which will contribute to a reduction in the cost of renewable energy used to generate green hydrogen.
Types of Hydrogen
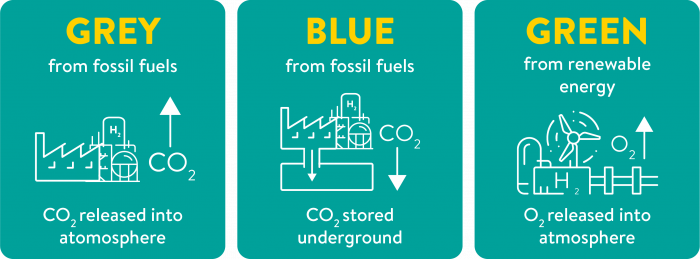
Hydrogen produced from fossil fuels (like coal, natural gas or methane) is called grey hydrogen, as it releases CO2 into the atmosphere contributing to climate-changing emissions.
However, if the CO2 from these fossil fuels is captured during the process and then stored safely, the impact of the process on the environment is much lower. Hydrogen produced in this manner is called blue hydrogen.
These CO2 emissions can be eliminated entirely from the production if the electricity used to produce hydrogen with electrolysis is supplied from renewable resources (such as solar or wind power). This type of hydrogen is called green hydrogen