Overview
Establishing alternative, efficient and stable electrocatalysts for the economical production of hydrogen from water is crucial for the net-zero transition.
This project, which was funded by Royce’s Industrial Collaboration Project (ICP), developed and tested different benchmarking approaches for earth-abundant, low-cost electrocatalysts, to facilitate the rapid translation of lab-scale discoveries into real-world devices.
About the Project
Ir-based oxides have so far proved to be the most viable oxygen evolution reaction (OER) catalysts, due to their combination of activity and stability. However, the scarcity and cost of iridium limits the scalability of water electrolysis technology.
Transition metal-based (TM-based) oxides provide a cheaper and more earth-abundant alternative, but are not stable in acidic electrolyte. As such, a shift towards developing catalysts for alkaline anion exchange membrane water electrolysers (AEM-WEs) has occurred.
Historically, it has been shown that common laboratory-scale methods for screening catalysts do not directly correspond to their performance in AEM-WEs. This discrepancy has been suggested to be caused by many factors including differences in mass transport and interfacial environment. Furthermore, variations in both structure and distribution of the electrocatalyst stemming from differing electrode preparations are also thought to be a contributing factor.
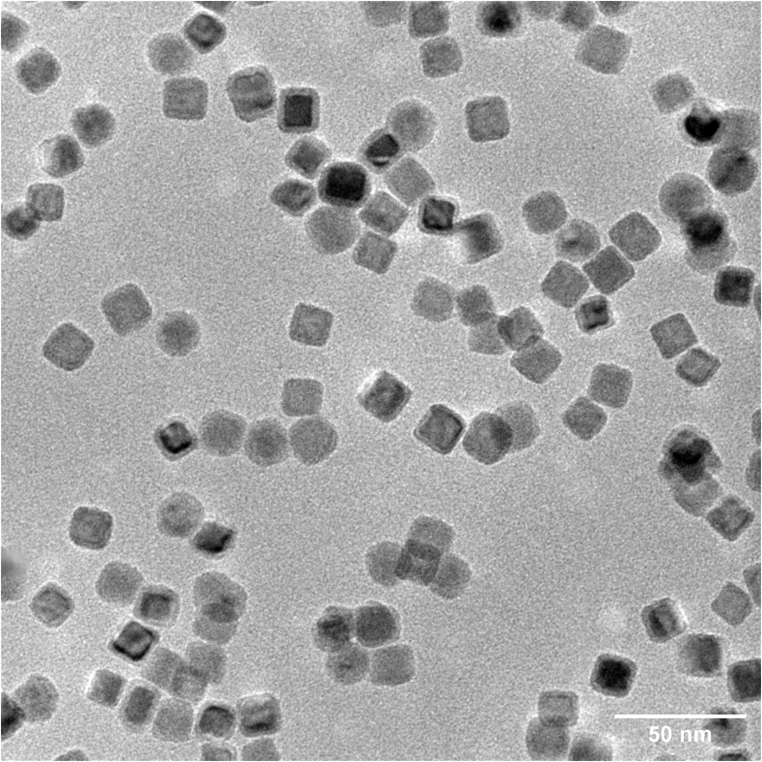
Therefore, the aim of this project was to benchmark comparable electrocatalysts in different electrode formats. The project started by determining a benchmark catalyst which was chosen to be TM-based NiFe2O4. Before depositing NiFe2Ox nanoparticles, commercial NiFe2O4 powder was used as a reference material for the electrochemical measurements.
Several new insights were gained during the project; in particular, concerning electrolyser measurements. Between MMU and NPL, rigorous measurements were performed in different electrolyser geometries and setups. This gave invaluable insights into how different laboratories may have differing results despite using identical catalysts, these setups included differing geometry, electrolyte flow rate, thickness of gaskets, and electrolyte temperature monitoring positions.
After testing the various setups, a final protocol was determined for OER catalysts. The most significant advance of the project was the first ever measurement of alkaline media catalysts using the floating electrode method. The floating electrode measurements were carried out on NiFe2O4 powder and extended to other Ni-based catalysts and compared with IrOx.
Nanoparticles of NiFe2Ox were successfully deposited onto various electrode formats; however, the adhesion onto the membrane was not as stable as the benchmark catalyst ink. On the other hand, the catalyst ink did not remain stable upon rotation forRDE measurements. This highlights the issue of electrode preparation in relation to the discrepancies found between methods.
Royce support was pivotal in the success of this project not just in the equipment available such as the nanoparticle deposition system and X-ray photoelectron spectrometer, but also the opportunities it provided for the groups and companies to collaborate. The project also resulted in an AEM-WE being commissioned at the University of Oxford. Once fully set up, the AEM-WE will be available through the Royce equipment access scheme.
The impact of this project includes a prospective publication on alkaline-based floating electrode measurements which will provide a methodology for the electrochemical community. Furthermore, the insights gained into the impact of electrolyser setup as well as catalyst preparation will be invaluable for future experimentdesign as well as understanding the discrepancies found within the literature.
The collaboration started with this project will alsocontinue, where investigations into the improvement of catalyst adhesion onto different electrodes will be one of the main focusses going forward.
Collaborators
The work was enabled through use of the facilities at the Henry Royce Institute and the University of Oxford, and due to a grant from the Henry Royce Institute’s Industrial Collaboration Programme.
""To produce hydrogen from renewable energy and water at the scale required for the net-zero transition, we need to develop low-cost electrocatalyst materials. However determining the performance of newly developed electrocatalysts is a major bottleneck. Importantly, this project developed and tested new methods for reliably and rapidly comparing electrocatalyst performance, so that lab-scale discoveries can be more rapidly scaled-up for future use in electrolyser devices.""
Robert Weatherup
Associate Professor, University of Oxford



